As4O6 (CM-7919) Structure

* Source: Asan Medical Center, Korea Institute of Radiological and Medical Science, CHA hospital, College of Veterinary Medicine, Seoul National University, Korea Institute of Science and Technology, Biotoxtech, Inje University, Catholic University of Korea, Handong University
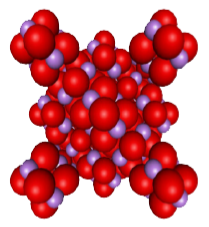
Molucular Weight: 395.6
Molecular Structure: Diamond Structure
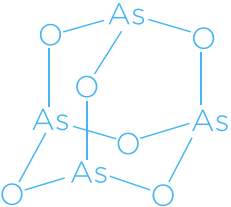
2,4,6,8,9,10 – Hexaoxa – 1,3,5,7 – tetraarsatricyclo[3.3.1.13,7]decane
As4O6 (Tetraarsenic hexaoxide) API

CM-7919 DP
< CM-7919 5mg Capsule >
Arsenic has long been used as a medicine
HISTORY OF ARSENIC
- Arsenic and arsenic compounds have been used as medicine or toxin for over 2000 years.
- Ointment containing arsenic is used as a therapeutic agent for dental disease, psoriasis, syphilis,
and rheumatic illness - Ancient Hippocrates age: Realgar (AS2S3) and orpiment (AS2S2) were used as a drug for ulcers, pest, malaria and various cancers
- Other historic records which demonstrate the use of arsenic:
– India: Pregnant women took it to improve the health of fetus
– Alps alpine region: Used as energy boosters
– India: Used as dermal ointment
– China: Physicians such as Jang Jung Gyeong, Pyeonjak, and Hwata used it to treat incurable diseases
– Korea: Heo Jun used arsenic to treat various incurable diseases - Used broadly as a medicinal product (to treat diseases) since the 18th century
Potassium carbonate solution of AS2O3 developed by Thomas Fowler(UK) in 1786 was used as a drug for rheumatism, seizures (epilepsy), ulcers, and indigestion. In 1910, the founder of chemotherapy, Paul Ehrlich(Germany) invented Salvarsan (compound 606) using arsenic which was widely used to treat syphilis and African trypanosomiasis. An organic arsenic compound, Melarsoprol, is currently being prescribed to treat trypanosomiasis invading CNS. From 1940s, it was used as a therapeutic agent for CML until novel treatment for CML was developed. - Reason for limited use of arsenic – ① Development of radiation therapy and other chemotherapies
② Known to be toxic with carcinogenic effects especially in long-term use - In 1992, Harbin medical University, China – Published the arsenic therapy for APL in an academic symposium in China
– Since Shen et al in China (Blood, 89; 3354-3360, 1999) reported that AS2O3 injections were effective in treating retinoic acid resistance in acute promyelocytic leukemia, a number of hospitals and researchers attempted to treat APL and various solid cancers with AS2O3 - In 1996, Shanghai Second Medical University published an article in American Society of Hematology
- In 2000, US FDA approved AS2O3 as an orphan medicinal product for the treatment of APL to all-trans retinoic acid
– Currently, arsenic is being studied in several countries including Korea and China - In Korea, CHEMAS has patent rights on As4O6 on its efficacy to prevent or treat solid tumors and coronavirus
Arsenic being used in contemporary cancer treatment
As2O3
- China – Reported in 1992 that AS2O3 is effective in treating APL
- US – In 2000, FDA approved AS2O3 as an orphan drug to treat APL
– TEVA’s “TRISENOX” is an injectable drug for intravenous administration (www.trisenox.com) - Still shows limited use of arsenic’s pharmaceutical potential and advantages
As4S4
- China – As4s4 is being used to treat CML and was proved by NMPA in 2009
- However, it cannot be used for a long period of time due to its toxicity.
- Other than the use for leukemia(APL, CML), its therapeutic effect on solid tumors has not yet been proved
As4O6 (CM-7919)
- Does not exhibit toxicity and adverse effects of arsenic compounds
- Exits human body in 2-3 days and is not accumulated in the body
- Almost no side effects even in long-term use
- Safety and effectiveness was proved in preclinical trials at Biotoxtech and phase I clinical trials at Asan Medical Center
- Huge therapeutic potential against various types of cancer
- Efficacy enhanced when combined with other conventional cancer treatments – chemotheraphy, radiotherapy, etc
- Shows efficacy in viral diseases (COVID-19, hepatitis B, etc)
